CBD Literature Review
The Role of Hemp-derived Phytocannabinoids in the Treatment of Inflammatory Conditions and Selected Psychiatric Disorders.
Philip Watkins Bsc.(Naturopathy)
Reviewed By Dr Vanessa Wong MBBS, MRCPsych, FHKCPsych, FHKAM (Psychiatry)
Introduction
Cannabis sativa has been an important source of food, fibre and medicine for thousands of years in the old world(1). Its achenes ("seeds") as well as other plant parts have been recorded in Chinese medical texts for nearly 2000 years. References to cannabis are found throughout classical Chinese literature, including many famous works of philosophy, poetry, agriculture, and medicine(2).In Europe, medications based on cannabis were used at the end of the 19th century to treat pain, spasms, asthma, sleep disorders, depression and loss of appetite(3).
In 1964, Dr Raphael Mechoulam made the first breakthrough in our understanding of the endocannabinoid system, where he elucidated the structure and stereochemistry of THC and CBD in his laboratory in Israel. These initial discoveries lead to the identification of many other cannabinoids(4). Post this discovery Dr Mechoulam was quoted as saying "I can't list all the physiological systems and conditions affected by cannabinoids because there are too many."
Currently, three general types of cannabinoids have been identified: phytocannabinoids present uniquely in the cannabis plant; endogenous cannabinoids produced in humans and animals; and synthetic cannabinoids gener- ated in a laboratory(5). There are over 550 chemical compounds and over 100 plant cannabinoids or phytocannabi- noids isolated from Cannabis sativa, including 69 - tetraydrocannabinol (THC) and cannabidiol (CBD)(6).
Another component of the endocannabinoid system is the prescence of the endogenous cannabinoid, anandamide. Named as such due to the Sanskrit term for "bliss" or "divine joy." Discovered in Israel in 1992, Lumir Hants, a Czech analytical chemist working in Israel with American pharmacologist William Devane, isolated anandamide, as the first known endocannabinoid in the human brain. Anandamide is either a neuromodulator or neurotransmitter that can play a role in pain, depression, appetite, memory and fertility(7).
The Endocannabinoid System and its relevance to the modern day
Cannabinoid receptors are distributed in the central nervous system (CNS) and many peripheral tissues, includ- ing the immune system, reproductive and gastrointestinal tracts, sympathetic ganglia, endocrine glands, arter- ies, lung and heart(8).
The CB1 and CB2 cannabinoid receptors are members of the G protein-coupled receptor (GPCR) family that were identified over 20 years ago(9). Around this time, the endocannabinoid system was defined as the ensemble of the following:
Two 7-transmembrane-domain and G protein-coupled receptors (GPCRs) for THC - cannabinoid receptor type-1 (CB1R) and cannabinoid receptor type-2 (CB2R);
Their two most studied endogenous ligands, the "endocannabinoids" N-arachidonoylethanolamine (anan- damide) and 2-arachidonolyglycerol (2-AG); and
The five enzymes considered, at the time, to be uniquely responsible for endocannabinoid biosynthesis (Refer Table 1)(10).
There is a broad variety of interactions with the CB1 receptor system and many different neurotransmitters and neuromodulators in the central and peripheral nervous system. For example, activation of the CB1 receptors leads to retrograde inhibition of the neuronal release of acetylcholine, dopamine, GABA, histamine, serotonin, glutamate, cholecystokinin, D-aspartate, glycine, and noradrenaline(11). Besides their involvement in controlling excitotoxicity and inflammation, compelling evidence shows that CB1 receptors in the CNS play an important role in neuroprotection(12).
CB2 receptors are identified peripherally in the circulating immune cells, the spleen(13)(14), and on macrophage-de- rived cells including osteocytes, osteoclasts, and hepatic Kupffer cells(15)(16), CB2 receptors can also be found in the enteric nervous system and colonic epithelium, where the endogenous ligands of CB receptors, anandamide and 2-AG, play an important role in the regulation of gastrointestinal motility, secretion, proliferation, and immune function(17)(18). The CB2 receptor has been shown to have potential as a therapeutic target in models of diseases, for example, neuropathic pain and neurodegenerative conditions such as Alzheimer's disease, where activation of the microglia and neuroinflammation are present(19).
Table 1

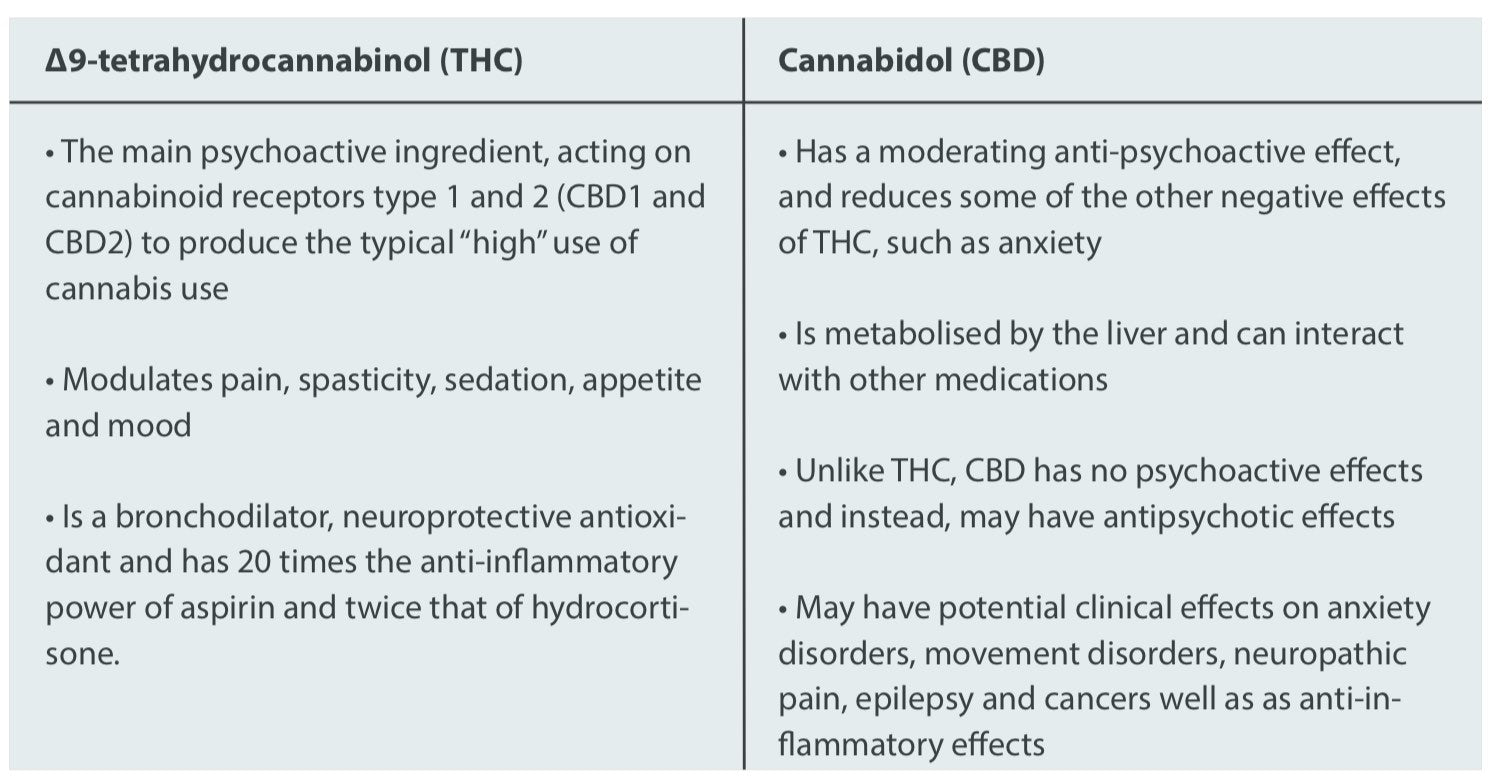
How do the actions of THC and CBD compare?
One of the more common misconceptions in this landscape lies in the differences between the major endocannabinoids. THC is a major constituent of Cannabis and serves as an agonist of the CB1 and CB2 receptors. CBD, the second major constituent of Cannabis, is virtually inactive at the CB1 receptor, and because of this negligible activity, it lacks the psychoactive effects that accompany the use of THC.
In addition, CBD has been demonstrated to antagonise some undesirable effects of THC, including intoxication, sedation, tachycardia and psychosis(21), while sharing protective, anti-oxidative, anti-emetic, and anti-carcinogenic properties (22)(23). Table 1 summarises some of these differences(24)(25).
Table 2
Broad-spectrum phytocannabinoids and their potential in pain management
Chronic pain represents an emerging public health issue of extreme proportions, particularly as a result of aging populations in industrialised countries. Associated facts and figures are unsettling: in Europe, chronic musculo- skeletal pain of a disabling nature affects over one in four people(26), while figures in Hong Kong suggest that chronic pain is a significant health problem in the adult population, with pain sufferers not being satisfied with the treatment they are receiving(27).
It is not just patients who are unsatisfied. Clinicians are faced with distinct difficulties when managing patients afflicted with intractable cancer-associated pain, neuropathic pain, and central pain states (pain associated with multiple sclerosis). Such conditions are often inadequately treated with available opiates, antidepressants and anticonvulsant drugs. Physicians are seeking new approaches to the treatment of these conditions but remain concerned about increasing government scrutiny of their prescribing practices(28) or prescription drug abuse.
The approach for pharmacological treatment for the relief of chronic pain is based primarily on pain intensity. This approach determines that mild pain should be treated with "simple" analgesics, whereas moderate to severe pain should be treated with opioids(29).
Interestingly, responses to an ABC news poll in the USA indicated that of the roughly 38 million people with chronic pain, 6% or an estimated 12 million people have utilised cannabis in attempts to treat it(30).
The Irish physician William Brooke O'Shaughnessy first introduced the analgesic effect of cannabis to the western world in a pioneer study in 1839(31). Any advancements in the development of medicinal cannabis has been impeded in the 20th century by prohibition in the majority of countries due to cannabis' psychoactive properties and potential for dependence. The psychotropic side effects (e.g. euphoria, anxiety, paranoia) or other CNS-relat- ed undesired side effects have also seemingly produced limitations for extended medicinal use.
Pharmacodynamics: Phytocannabinoids act on multiple pain targets
Originally, it was thought that the CB1 receptors were found predominantly in the CNS, and CB2 receptors were found predominantly in cells involved with immune function(32). However, recently this has become much more complicated, as it has been recognised that cannabinoids, both plant-derived and endogenous, act simultaneously on multiple pain targets(33)(34)(35)(36)(37)(38) within the peripheral and CNS.
Beside acting on cannabinoid CB1/CB2 receptors they may reduce pain through interaction with the putative non CB1/CB2 cannabinoid G protein-coupled receptor (GPCR) 55 (GPR55) or GPCR 18 (GPR18), also known as the
N-arachidonoyl glycine (NAGly) receptor and other well-known GPCRs such as opioid or serotonin (5-HT) receptors(41)(42).
CBD also regulates the perception of pain by affecting the activity of a significant number of other targets• includ- ing non-cannabinoid GPCRs (e·g· 5-HT1A), ion channels (TRPV1, TRPA1 and TPRM8, GlyR), PPARs, while also inhibiting uptake of AEA and weakly inhibiting its hydrolysis by the enzyme fatty acid amide hydrolase (FAAH)(43)(44)(45)(46)(47)(48).
Several lines of evidence indicate that cannabinoids such as CBD and THC may contribute to pain relief through an anti-inflammatory action(49)(50). In addition• non-cannabinoid constituents of the cannabis plant that belong to miscellaneous groups of natural products (terpenoids and flavonoids) may contribute to the analgesic, as well as the anti-inflammatory effect of cannabis(51)(52).
Further animal and genetic models suggest that the mechanisms of the analgesic effect of cannabinoids include inhibition of the release of neurotransmitters and neuropeptides from presynaptic nerve endings, modulation of postsynaptic neutron excitability, activation of descending inhibitory pain pathways, and reduction of neuronal inflammation(53).
Table 3: Anti-inflammatory effects and pain management with phytocannabinoids and CBD:

Although CBD is still to be evaluated clinically as a monotherapy for pain, its anti-inflammatory(54) and anti-spas-modic benefits and good safety profile suggest that it could be an effective and safe analgesic(55). At this point there is moderate-quality evidence to support the use of cannabinoids for the treatment of chronic pain and spasticity(56).
Our knowledge relating to the contribution of cannabinoids and the attenuation of central sensitisation by the activation of CB2 receptors in osteoarthritis sees a therapeutic potential for the use of CBD and other cannabinoids such as THC and CBN for the treatment of osteoarthritis also(57).
Central sensitisation plays a pivotal role in the switch from acute to chronic pain mechanisms(58)(59), and the manifestation of altered sensory responses, such as touch-evoked pain (mechanical allodynia), in models of chronic pain(60). The discovery of a contribution of central sensitisation to osteoarthritic pain supports the investigation of novel molecular targets within the CNS and the peripheral nervous system for the treatment of osteoarthritic pain. The analgesic effects produced by activation of the cannabinoid receptor system are well documented and mediated by multiple sites of action(61).
The joint pain, often originating from central sensitisation, can often be characterised by increases in the activity of metalloproteases MMP-2 and MMP-9 in the spinal cord(62). CB2 agonists exert their analgesic effects in these osteoarthritis models by attenuating the activity of these enzymes(63).
In mouse models, CBD and THC decrease the production and release of proinflammatory cytokines, including interleukin1B, interleukin-6, and interferon (IFN)β, from lipopolysaccahride-activated microglial cells. In addition, CBD, not THC, reduces the activity of the NF-κB pathway- a primary pathway regulating the expression of pro-in- flammatory genes(64).
Other promising conditions benefitting from the use of phytocannabinoids include neuropathic pain and cancer-related pain. Cannabis-based medicinal extracts used in different populations of chronic non-malignant neuropathic pain patients may provide effective analgesia in conditions that are refractory to other treatments(65).
Phytocannabinoids are also approved in many countries as an adjunctive analgesic in those with advanced cancer and malignant diseases where standard opioid treatment is ineffective. A systematic review of literature indicated that cannabinoid therapy reduced pain intensity by >30% in those with malignant diseases(66).
Table 4

CBD and the treatment of neurodegenerative disorders
Microglial activation and neuroinflammation appear to be the upstream mechanism underlying the pathogene- sis of neurodegenerative diseases, including neuropathic pain, Alzheimer's disease, Parkinson's disease, multiple sclerosis, amyotrophic lateral sclerosis, AIDS and Huntington's disease(67)(68)(69)(70)(71)(72)(73).
CBD is a well-known antioxidant, exerting neuroprotective actions that might be relevant to the treatment of neurodegenerative diseases, including Alzheimer's disease, Parkinson's Disease and Huntington's Disease(74).
CBD exerts an anti-apoptotic effect against the neuronal damage induced by the β-amyloid peptide (AB). It inhib- its AB-induced neurotoxicity in PC12 cells and this effect is mediated by the WNT-B-catenin pathway(75), an import- ant finding in light of the observation that disruption of the WNT pathway by AB represents a pivotal event in the neuronal apoptosis occurring in Alzheimer's disease. CBD may also prove beneficial in preventing apoptotic signalling in neurons via restoration of Ca2+ homeostasis(76).
Several clinical studies have shown CBD as an effective treatment modality for reducing psychotic symptoms, improving dystonic symptoms, diminishing events related to REM sleep behaviour disorders, and improving quality of life in movement disorders such as Parkinson's disease(77).
CBD in anxiety-related conditions
Existing preclinical evidence strongly supports CBD as a treatment for generalised anxiety disorder, panic disorder, social anxiety disorder, obsessive-compulsive disorder, and post-traumatic stress disorder when administered acutely(78).
Interestingly, a single dose of CBD not only significantly decreased subjective anxiety symptoms(79), but also reduced cognitive impairment, speech performance discomfort and alertness in anticipatory speech during a Simulation Public Speaking Test(80), in comparison to the placebo group.
It is believed that the acute anxiolytic effect of CBD in individuals with generalised social anxiety disorder comes from its modification of cerebral blood flow in limbic and paralimbic areas, and as a 5-HT1A receptor agonist in animal models(81).
The 5-HT1AR is an established anxiolytic target. Buspirone and other 5-HT1AR agonist are approved for the treat- ment of generalised anxiety disorder with fair response rates(82). In preclinical studies, 5-HT1AR agonists are anxio- lytic in animal models of general anxiety(83), prevent the adverse effects of stress(84), and enhance fear extinction(85). This leads the way for CBD to be further developed as a natural alternative for those wishing to follow that route.
This same activation of the 5-HT1AR has CBD improving several symptoms associated with post-traumatic stress disorder, including reducing acute heart rate and blood pressure, delaying 24- hour antigenic effects of stress, reducing arousal and avoidance, and enhancing the extinction and blocking the reconsolidating of persistent fear memories(86).
Interactions between CBD and other phytocannabindoids with CYP450 Enzymes
Cytochrome P450 (CYP) enzymes are harm-containing mono-oxygenases bound to the membranes of the endoplasmic reticulum or mitochondria in the liver, intestine, kidney, lung, brain, skin, and heart, with the highest level of expression in the liver and intestine(87)(88). Variability in the drug plasma levels may diverge depending on differ- ent factors, and according to some authors, may reach up to 40-fold differences(89). The most important factors influencing drug plasma levels include the activities of the CYP's with their genetic polymorphisms, epigenetic changes such as DNA methylation and histone deacetylation, together with exogenous factors. These factors substantially influencing CYP metabolic activity are the major source of variability in the pharmacokinetics of drugs and thus in drug responses(90). CYPs are therefore of particular relevance in clinical pharmacokinetics. CYPs are also involved in the metabolism of endogenous substances, most notably in this case, endocannabinoids(91).
Whilst THC, CBD and CBN are the most studied substances of the phytocannabinoid group in terms of CYP inter- actions. All three competitively inhibit the CYP1A group, but with different strengths. CBD is considered to be the most potent inhibitor of the CYP enzyme family, in particular., depending on the specific enzyme in the family(92).
A potential mechanism of action for this interaction may lie within the broader influence the endocannabinoids system has in the CNS. It is known that the genes coding for various CYPs are regulated by endogenous hormones within the CNS but hepatic CYP expression has also been shown to be influenced by changes in the brain dopaminergic, noradrenergic, and serotonergic systems(93). The central endocannabinoid system modulates neurotransmission at inhibitory and excitatory synapses, and therefore could also be involved in the regulation of CYP activity.
On the basis of some of these preliminary findings, in November 2019, the FDA tightened its recommendations regarding the use of commercially oriented CBD with further research and caution recommended when using CBD and other phytocannabinoids such as THC and CBN concomitantly with other medications that impact the liver(94).
Conclusion
Preclinical and animal model studies in the endocannabinoid system have found numerous ways which phyto- cannabinoids such as THC and CBD, can expand on therapeutic options for conditions either in combination or independently of modern pharmaceutical applications via their influence on multiple molecular targets.
As more human clinical studies are completed, the potential of phytocannabinoids and their use in pain, mental health and other classically inflammatory conditions is clear and exciting. It is thought important to note that the utilisation of CBD and other phytocannabinoids and their interactions with other medications must be defined clearly in order reduce potential harm for the patient, especially those with preexisting conditions.
Appendix 1: Summary of biologically active chemical compounds found in broad-specrum CBD

References:
1 Callaway J (2004). Hempseed as a nutritional resource: an overview. Euphytica 140: 65–72.
2 Brand EJ and Zhao Z (2017) Cannabis in Chinese Medicine: Are Some Traditional Indications Referenced in Ancient Literature Related to Cannabinoids? Front. Pharmacol. 8:108. doi: 10.3389/fphar.2017.00108
3 Fankhauser M: Cannabis in der westlichen Medizin. In: Groten- hermen F (ed.): Cannabis und Cannabinoide. Pharmakologie, Toxi- kologie und therapeutisches Potential. 2nd edition. Göttingen: Hans Huber 2004; 57–71.
4 Pertwee, Roger (2006), Cannabinoid pharmacology: the first 66 years, British Journal of Pharmacology (2006) 147, S163–S171. doi:10.1038/sj.bjp.0706406
5 Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for cancer treatment: progress and promise. Cancer Res. 2008; 68:339–342. [PubMed: 18199524]
6 ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. Phytochemistry of Cannabis sativa LPhytocannabinoids. A. Douglas Kinghorn, Heinz Falk, Simon Gibbons, Jun'ichi Kobayashi (eds). Springer: Switzerland, 2017, pp 1–36.
7 Lambert, Didier, “Cannabinoids in Nature and Medicine, August 2009, Wiley Publishers
8 Franjo Grotenhermen, “ Cannabinoids”, Current Drug Targets - CNS & Neurological Disorders (2005) 4: 507. https://- doi.org/10.2174/156800705774322111
9 Russo EB, Marcu J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In: Advances in Pharmacology. Elsevier; 2017. p. 67–134.
10 Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant - do they exist?. Br J Pharmacol. 2010;160(3):523–529. doi:10.1111/j.1476-5381.2010.00745.x
11 Grotenhermen F, Müller-Vahl K: The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int 2012; 109(29–30): 495–501. DOI: 10.3238/arztebl.2012.0495
12 Sanchez AJ, Garcia-Merino A. Neuroprotective agents: cannabinoids. Clin Immunol. 2011; 142:57– 67. [PubMed: 21420365]
13 Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993; 365:61–5. [PubMed: 7689702]
14 Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid recep- tors in human immune tissues and leukocyte subpopulations. European journal of biochemistry / FEBS. 1995; 232:54–61.
15 Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proceed- ings of the National Academy of Sciences of the United States of America. 2006; 103:696–701. [PubMed: 16407142]
16 Louvet A, Teixeira-Clerc F, Chobert MN, Deveaux V, Pavoine C, Zimmer A, et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology (Baltimore, Md). 2011; 54:1217–26.
17 Fichna J, Bawa M, Thakur GA, Tichkule R, Makriyannis A, et al. (2014) Cannabinoids Alleviate Experimentally Induced Intestinal Inflammation by Acting at Central and Peripheral Receptors. PLoS ONE 9(10): e109115. doi:10.1371/journal.pone.0109115
18 Izzo AA, Sharkey KA (2010) Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther 126: 21–38.
19 Bie B, Wu J, Foss JF, Naguib M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr Opin Anaesthesiol. 2018;31(4):407–414. doi:10.1097/ACO.0000000000000616
20 Jeet, Rimple & Ambwani, Sneha & Singh, Surjit. (2016). Endocannabinoid System: A Multi-Facet Therapeutic Target. Current clinical pharmacology. 11. 10.2174/1574884711666160418105339.
21 Zuardi, A.W., Shirakawa, I., Finkelfarb, E. et al. Action of cannabidiol on the anxiety and other effects produced by δ9-THC in normal subjects. Psychopharmacology 76, 245–250 (1982). https://doi.org/10.1007/BF00432554
22 Pellati, F., Borgonetti, V., Brighenti, V., Biagi, M., Benvenuti, S., & Corsi, L. (2018). Cannabis sativa L. and Nonpsychoactive Cannabi- noids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed research international, 2018, 1691428. doi:10.1155/2018/1691428
23 Vucˇkovic S,SrebroD,Vujovic KS, Vucˇetic CˇandProstranM(2018) Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 9:1259. doi: 10.3389/fphar.2018.01259
24 Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M. Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and 69-Tetrahydrocannabinol. Neuropsychopharmacology. 2018 Jan;43(1):142–54.
25 Russo EB, Marcu J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In: Advances in Pharmacology. Elsevier; 2017. p. 67–134.
26 Frondini C, Lanfranchi G, Minardi M, et al. 2007. Affective, behaviour and cognitive disorders in the elderly with chronic musculo- skelatal pain: the impact on an aging population. Arch Gerontol Geriatr, 44(Suppl 1):167–71.
27 Ng JKF, Tsui SL, Chan WS. Prevalence of common chronic pain in Hong Kong adults. Clin J Pain. 2002;18:275–281. doi: 10.1097/00002508-200209000-00001.
28 Fishman SM. 2006. Pain and politics: DEA, Congress, and the courts, oh my! Pain Med, 7:87–8.
29 Breivik H, Collett B, Ventafridda V, Co- hen R, Gallacher D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain 2006; 10:287-287.
30 ABC News, USA Today, Stanford Medical Center Poll. 2005. Broad experience with pain sparks search for relief [online]. URL: http://abcnews. go.com/images/Politics/979a1TheFightAgainstPain.pdf.
31 O’Shaughnessy W. Case of tetanus, cured by a preparation of hemp (the cannabis indica). In: O’Shaughnessy W (ed). Transactions of the Medical and Physical Society of Bengal, On the prepa- rations of Indian hemp. Calcutta Bishops Cotton Press 1839, pp 1838-1840.
32 Rahn, E. J., and Hohmann, A. G. (2009). Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics 6, 713–737. doi: 10.1016/j.nurt.2009.08.002
33 Vučković S, Srebro D, Vujović KS, Vučetić Č and Prostran M (2018) Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 9:1259. doi: 10.3389/fphar.2018.01259
34 Ross, R. A. (2003). Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 40, 790–801. doi: 10.1038/sj.bjp.0705467
35 Horvath, G., Kekesi, G., Nagy, E., and Benedek, G. (2008). The role of TRPV1 receptors in the antinociceptive effect of anandamide at spinal level. Pain 134, 277–284. doi: 10.1016/j.pain.2007.04.032
36 Pertwee, R. G., Howlett, A. C., Abood, M. E., Alexander, S. P., Di Marzo, V., Elphick, M. R., et al. (2010). International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 62, 588–631. doi: 10.1124/pr.110.003004
37 O’Sullivan, S. E. (2016). An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 173, 1899–1910. doi: 10.1111/bph.13497
38 Morales, P., Hurst, D. P., and Reggio, P. H. (2017). Molecular targets of the phytocannabinoids-a complex picture. Prog. Chem. Org. Nat. Prod. 103, 103– 131. doi: 10.1007/978-3-319-45541-9_4
39 Staton, P. C., Hatcher, J. P., Walker, D. J., Morrison, A. D., Shapland, E. M., Hughes, J. P., et al. (2008). The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 139, 225–236. doi: 10.1016/j.pain.2008.04.006
40 Hwang, J., Adamson, C., Butler, D., Janero, D. R., Makriyannis, A., and Bahr, B. A. (2010). Enhancement of endocannabinoid signaling by fatty acid amidehydrolase inhibition: a neuroprotective therapeutic modality. Life Sci. 86, 615– 623. doi: 10.1016/j.lfs.2009.06.003
41 Russo, E. B., Burnett, A., Hall, B., and Parker, K. K. (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 30, 1037–1043. doi: 10.1007/ s11064- 005- 6978- 1
42 Scavone, J. L., Sterling, R. C., and Van Bockstaele, E. J. (2013). Cannabinoid and opioid interactions: implications for opiate depen- dence and withdrawal. Neuroscience 248, 637–654. doi: 10.1016/j.neuroscience.2013.04.034
43 Russo, E. B., Burnett, A., Hall, B., and Parker, K. K. (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 30, 1037–1043. doi: 10.1007/ s11064- 005- 6978- 1
44 Staton, P. C., Hatcher, J. P., Walker, D. J., Morrison, A. D., Shapland, E. M., Hughes, J. P., et al. (2008). The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 139, 225–236. doi: 10.1016/j.pain.2008.04.006
45 Ahrens, J., Demir, R., Leuwer, M., de la Roche, J., Krampfl, K., Foadi, N., et al. (2009). The nonpsychotropic cannabinoid cannabidiol modulates and directly activates alpha-1 and alpha-1-Beta glycine receptor function. Pharmacology 83, 217–222. doi: 10.1159/000201556
46 De Petrocellis, L., Ligresti, A., Moriello, A. S., Allarà, M., Bisogno, T., Petrosino, S., et al. (2011). Effects of cannabinoids and cannabi- noid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x
47 Burstein, S. (2015). Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg. Med. Chem. 23, 1377–1385. doi: 10.1016/j.bmc.2015.01. 059
48 Morales, P., Hurst, D. P., and Reggio, P. H. (2017). Molecular targets of the phytocannabinoids-a complex picture. Prog. Chem. Org. Nat. Prod. 103, 103– 131. doi: 10.1007/978-3-319-45541-9_4
49 Jesse Lo, V., Fu, J., Astarita, G., La Rana, G., Russo, R., Calignano, A., et al. (2005). The nuclear receptor peroxisome proliferator-activat- ed receptor- alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 67, 15–19. doi: 10.1124/mol.104.006353
50 Klein, T. W. (2005). Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 5, 400–411. doi: 10.1038/n- ri1602
51 Andre, C. M., Hausman, J. F., and Guerriero, G. (2016). Cannabis sativa: the plant of the thousand and one molecules. Front. Plant Sci. 7:19. doi: 10.3389/fpls.2016. 00019
52 ElSohly, M. A., Radwan, M. M., Gul, W., Chandra, S., and Galal, A. (2017). Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 103, 1–36. doi: 10.1007/978-3-319-45541-9_1
53 Vučković S, Srebro D, Vujović KS, Vučetić Č and Prostran M (2018) Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 9:1259. doi: 10.3389/fphar.2018.01259
54 Ko, G. D., Bober, S. L., Mindra, S., and Moreau, J. M. (2016). Medical cannabis - the Canadian perspective. J. Pain Res. 9, 735–744. doi: 10.2147/JPR.S98182
55 Wade, D. T., Robson, P., House, H., Makela, P., and Aram, J. (2003). A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin. Rehabil. 17, 21–29. doi: 10. 1191/0269215503cr581oa
56 Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015;313(24):2456–2473. doi:https://doi.org/10.1001/jama.2015.6358
57 Burston JJ, Sagar DR, Shao P, Bai M, King E, et al. (2013) Cannabinoid CB2 Receptors Regulate Central Sensitization and Pain Responses Associated with Osteoarthritis of the Knee Joint. PLoS ONE 8(11): e80440. doi:10.1371/journal.pone.0080440
58 Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139: 267–284.
59 Woolf CJ, Salter MW (2000) Neuronal plasticity: increasing the gain in pain. Science 288: 1765–1769.
60 Sandkuhler J (2009) Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 89: 707–758.
61 Sagar DR, Burston JJ, Woodhams SG, Chapman V (2012) Dynamic changes to the endocannabinoid system in models of chronic pain. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 3300–3311.
62 Burston JJ, Sagar DR, Shao P, Bai M, King E, et al. (2013) Cannabinoid CB2 Receptors Regulate Central Sensitization and Pain Responses Associated with Osteoarthritis of the Knee Joint. PLoS ONE 8(11): e80440. doi:10.1371/journal.pone.0080440
63 Burston JJ, Sagar DR, Shao P, Bai M, King E, et al. (2013) Cannabinoid CB2 Receptors Regulate Central Sensitization and Pain Responses Associated with Osteoarthritis of the Knee Joint. PLoS ONE 8(11): e80440. doi:10.1371/journal.pone.0080440
64 Kozela E., Pietr M., Juknat A., Rimmerman N., Levy R., Vogel Z. Cannabinoids 69-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 2010;285:1616–1626. doi: 10.1074/jbc.M109.069294.
65 Boychuk, Darrell & Goddard, Greg & Mauro, Giovanni & Orellana, Maria. (2015). The Effectiveness of Cannabinoids in the Manage- ment of Chronic Nonmalignant Neuropathic Pain: A Systematic Review. Journal of Oral & Facial Pain and Headache. 29. 7-14.10.11607/ofph.1274.
66 Darkovska Serafimovska, Marija & Serafimovska, Tijana & Arsova-Sarafinovska, Zorica & Stefanoski, Sasho & Keskovski, Zlatko & Balkanov, Trajan. (2018). Pharmacotherapeutic considerations for use of cannabinoids to relieve pain in patients with malignant diseases. Journal of Pain Research. Volume 11. 837-842. 10.2147/JPR.S160556.
67 Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003; 23:11136–41. [PubMed: 14657172]
68 Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, et al. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus- induced encephalitis. J Neurosci. 2005; 25:2530–6. [PubMed: 15758162]
69 Chung YC, Shin WH, Baek JY, Cho EJ, Baik HH, Kim SR, et al. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Experimental & molecular medicine. 2016; 48:e205. [PubMed: 27534533]
70 Naguib M, Xu JJ, Diaz P, Brown DL, Cogdell D, Bie B, et al. Prevention of paclitaxel-induced neuropathy through activation of the central cannabinoid type 2 receptor system. Anesth Analg. 2012; 114:1104–20. [PubMed: 22392969]
71 Wu J, Bie B, Yang H, Xu JJ, Brown DL, Naguib M. Activation of the CB(2) receptor system reverses amyloid-induced memory deficien- cy. Neurobiology of aging. 2013; 34:791–804. [PubMed: 22795792]
72 Wu J, Hocevar M, Foss JF, Bihua Bie B, Naguib M. Activation of CB2 receptor system restores cognitive capacity and hippocampal Sox2 expression in a transgenic mouse model of Alzheimer’s disease. European journal of pharmacology. 2017:811. The authors demonstrated that activation of CB2 receptor suppressed the neuroinflammation, restored the neurogenesis, and rescued the hippocampal glutamatergic plasticity and cognition in the transgenic mice model of Alzheimer’s disease.
73 Yamamoto W, Mikami T, Iwamura H. Involvement of central cannabinoid CB2 receptor in reducing mechanical allodynia in a mouse model of neuropathic pain. European journal of pharmacology. 2008; 583:56–61. [PubMed: 18279850]
74 Izzo A.A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006.
75 Esposito, G. et al. (2006) The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J. Mol. Med. 84, 253–258
76 Drysdale, A.J. et al. (2006) Cannabidiol-induced intracellular Ca2+ elevations in hippocampal cells. Neuropharmacology 50, 621–631
77 Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH and Abílio VC (2018) Cannabidiol as a Promising Strategy to Treat and Prevent Movement Disorders? Front. Pharmacol. 9:482. doi: 10.3389/fphar.2018.00482
78 Blessing, E.M., Steenkamp, M.M., Manzanares, J. et al. Cannabidiol as a Potential Treatment for Anxiety Disorders Neurotherapeutics (2015) 12: 825. https://doi.org/10.1007/s13311-015-0387-1
79 Crippa JAS, Nogueira Derenusson G, Borduqui Ferrari T, Wichert-Ana L, Duran FLS, Martin-Santos R, Vinícius Simões M, Bhattacha- ryya S, Fusar-Poli P, Atakan Z, Santos Filho A, Freitas-Ferrari MC, McGuire PK, Zuardi AW, Busatto GF, Hallak JEC (2011). Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. Journal of Psychopharmacology 25, 121–130.
80 Bergamaschi MM, Queiroz RHC, Chagas MHN, De Oliveira DCG, De Martinis BS, Kapczinski F, Quevedo J, Roesler R, Schröder N, Nardi AE, Martín-Santos R, Hallak JEC, Zuardi AW, Crippa JAS (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-nave social phobia patients. Neuropsychopharmacology 36, 1219.
81 Mandolini, G., Lazzaretti, M., Pigoni, A., Oldani, L., Delvecchio, G., & Brambilla, P. (2018). Pharmacological properties of cannabidiol in the treatment of psychiatric disorders: A critical overview. Epidemiology and Psychiatric Sciences, 27(4), 327-335. doi:10.1017/S2045796018000239
82 Chessick CA, Allen MH, Thase M, et al. Azapirones for general- ized anxiety disorder. Cochrane Database Syst Rev 2006;CD006115.
83 Roncon CM, Biesdorf C, Coimbra NC, et al. Cooperative regula- tion of anxiety and panic-related defensive behaviors in the rat periaqueductal grey matter by 5-HT1A and mu-receptors. J Psychopharmacol 2013;27:1141-1148.
84 Zhou J, Cao X, Mar AC, et al. Activation of postsynaptic 5-HT1A receptors improve stress adaptation. Psychopharmacology 2014;231:2067-2075.
85 Saito Y, Matsumoto M, Yanagawa Y, et al. Facilitation of fear extinc- tion by the 5-HT(1A) receptor agonist tandospirone: possible in- volvement of dopaminergic modulation. Synapse 2013;67:161-170.
86 Blessing, E.M., Steenkamp, M.M., Manzanares, J. et al. Cannabidiol as a Potential Treatment for Anxiety Disorders Neurotherapeutics (2015) 12: 825. https://doi.org/10.1007/s13311-015-0387-1
87 Hasler, J.A.; Estabrook, R.M.M.; Pikuleva, I.; Waterman, M.; Capdevila, J.; Holla, V.; Helvig, C.; Falck, J.R.; Farrell, G.; Ka- minsky, L.S.; Spivack, S.D.; Boitier, E.; Beaune, P. Human cyto- chromes P450. Mol. Aspects Med., 1999, 20(1-2), 1-137.
88 Anzenbacher, P.; Anzenbacherová, E. Cytochromes P450 and metabolism of xenobiotics. Cell. Mol. Life Sci., 2001, 58(5-6), 737- 747.
89 Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther., 2013, 138(1), 103- 141.
90 Zendulka, Ondrej & Dovrtělová, Gabriela & Noskova, Kristyna & Turjap, Miroslav & Sulcova, Alexandra & Hanus, Lumir & Jurica, Jan. (2015). Cannabinoids and Cytochrome P450 Interactions. Current drug metabolism. 17. 10.2174/1389200217666151210142051.
91 Zelasko, S.; Arnold, W.R.; Das, A. Endocannabinoid metabolism by cytochrome P450 monooxygenases. Prostaglandins Other Lipid Mediat., 2015, 116-117, 112-123.
92 Yamaori, S.; Kushihara, M.; Yamamoto, I.; Watanabe, K. Charac- terization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 en- zymes. Biochem. Pharmacol., 2010, 79(11), 1691-1698.
93 Wójcikowski, J.; Daniel, W.A. The role of the nervous system in the regulation of liver cytochrome p450. Curr. Drug. Metab., 2011, 12(2), 124-138.
94 https://www.fda.gov/consumers/consumer-up- dates/what-you-need-know-and-what-were-working-find-out-about-products-containing-cannabis-or-cannabis