Digestive Dysfunction Dysbiosis – an example caused by Helicobacter pylori
Dysbiosis caused by lack of digestive enzymes is common. For instance, one meta-analysis of over twenty studies looking for the incidence of atrophic gastritis in a wide population age group found a 27% incidence in 4241 subjects. They were assessed for atrophic gastritis with a serum panel combination of pepsinogen, gastrin-17 and anti-H. pylori antibodies. [1].
Atrophic gastritis is a process of chronic inflammation of the gastric mucosa of the stomach, leading to a loss of gastric glandular cells and their eventual replacement by intestinal and fibrous tissues. As a result, the stomach's secretion of hydrochloric acid, pepsin, and intrinsic factor is impaired, potentially leading to malabsorption, digestive dysfunction and intestinal dysbiosis [2], as will be discussed further below. In humans the degree of gastric inflammation and bacteria in the upper intestine (SIBO) is greater when H pylori overgrowth is more prominent, which may simply reflect the greater degree of hypochlorhydria in patients with H pylori-induced inflammation. [3].
Most patients chronically infected with H. pylori manifest a pangastritis with reduced acid secretion due to bacterial virulence factors, inflammatory cytokines, and various degrees of gastric atrophy. [4,5].

PPI use gives a good indication of the type of dysbiosis caused by stomach acid suppression. PPI’s significantly increased the presence of Streptococcaceae and Enterococcaceae, and also decreased that of Faecalibacterium, [6]. A meta-study on PPI use confirms the Streptococcal finding and found that PPI use was associated with several other distinct taxonomic alterations in the small intestine. This meta-study especially showed overgrowth of orally derived bacteria – Streptococci. In fecal samples, PPIs increased Streptococci, Staphylococci, Enterococci, Lactobacillus, and Enterobacter. Decreased by PPIs were beneficial Bifidobacteria, and butyrate producing members in the Clostridia genus. [7].
In our clinical example, who was a female client of 46 years of age presenting with excessive gas and bloating, fullness after a meal, and altered bowel frequency, predominantly with constipation as symptoms. In addition her BMI was 28 with abdominal obesity suggesting a tendency to metabolic syndrome. She also had a previous blood test from a year prior indicating low ferritin of 14 and she presented with fatigue symptoms suggestive of anemia. Her MCV had been 92 a year prior, probably indicating lower B12 levels than ideal.

Page 1 showed no pathogens.
Note that in her GI Map a low level of H pylori is found – and this is commonly encountered since over half Hong Kong patients harbor the bacteria. It is a non-virulent H pylori strain at just 410 (4.10e2) per gram of stool.
Could this be relevant to her dysbiosis?
Several species associated with hypochlorhydria are present: Enterobacter and Enterococcus. Also the two major phylum Bacteroidetes and Firmicutes are both elevated – indicating bacterial overgrowth in the colon is significant. In the following section of this GI Map we see Streptococci, Staphylococci, and the Enterococcus species in overgrowth, and again these associate with hypochlorhydria, based on the PPI studies.

The point being made is that just as PPI’s can induce SIBO, [8], so can both atrophic gastritis and the hypochlordydria induced by H pylori. [9].
In this case the patient had also undergone a SIBO breath test as most symptoms of her bloating and discomfort were at the umbilical region, and were not epigastric. Also she rarely had reflux except after alcohol at night. Her symptoms had been classified by her MD as IBS-M, though it was primarily constipation with occasional days of several stools in quick succession as a clearance. We can see from the breath test, she has SIBO, one with methane as well as hydrogen.
SIBO Breath Test

The next critical step in how digestive insufficiency dysbiosis then affects other visceral organs following SIBO is evident here as well. In our patients report below, her Elastase 1 is at 216 micg/g indicating low lipase, amalase and the protesase trypsin levels from her pancreas, or pancreatic exocrine insufficiency. This is adding to undigested food feeding her bacterial overgrowth.

Can SIBO affect the pancreas?
In chronic pancreatitis versus controls with no SIBO, there was a 5.58x increase in chronic pancreatitis in those with SIBO. [10]. A positive association between the presence of SIBO and pancreatic enzyme replacement therapy use reached significance (p=0.052) in chronic pancreatitis patients. [11].
Most SIBO patients trend in the direction of low Elastase 1 (<500 - commonly in the 220-350 micg/g range). This indicates a moderate pancreatic exocrine insufficiency. If there is chronic pancreatitis then Elastase 1 level would be found under 200micg/g, and averages 100micg/g. [12], which is when pancreatic enzyme replacement therapy is given medically. In the latter study normal Elastase 1 is established as 500 micg/g in individuals with normal healthy pancreatic ultrasound.
Digestive Dysfunction and Bacterial Fermentation
On another important theme, hypochlorhydria, atrophic gastritis and pancreatic exocrine insufficiency lead to malabsorption of amino acids as the proteins are less digested. This promotes bacterial fermentation especially in the colon where normally proteins are rare, unless they are over consumed in the diet.
In the meat over-consumers, the excess protein has been shown to promote Escherichia coli, Bacteroides, Prevotella, Allstipes, and Clostridium cluster XIVa all of which can utilize protein. [13]. The E. coli is also associated with SIBO. [14]. Clinically high levels of E. coli in a GI Map provide a good hint that protein excess is available in the gut, either from the diet or from protein maldigestion.
Protein catabolism in the gut generally has a negative connotation, as by-products that are toxic to the host can result from this process, including amines, phenols, ammonia, and sulfurous compounds, notably H2S. The aptly named putrescine and cadaverine are also derived from amino acid metabolism by microbes, and account for bad breath and body odor.
Overall proteins availability in the colon favors a potentially pathogenic and pro-inflammatory microbiota profile. These metabolites largely compromise the colonic epithelium structure, causing mucosal inflammation, but may also directly modulate the enteric nervous system and intestinal motility. [15].
However, the most abundant end products of bacterial fermentation of proteins are the SCFA fuels, favoring the overgrowth of the colonic microbiome. [16]. In a GI Map we see this as many high counts of the above (Escherichia coli, Bacteroides, Prevotella), and for the phylum Bacteroidaceae and/or Firmicutes, as seen in our patients report.
The thesis presented here is that the digestive dysfunction dysbiosis can be induced by the H pylori, even if at low levels. The associated hypochlorhydria, and potentially the development of atrophic gastritis induce SIBO and GI fermentation with excessive intestinal toxin production. This may increase incidence of inflamed and degenerating function of the liver and pancreas, quite apart from any systemic effects.
Our patient had a history of low iron and B12, is this also related?
Human subjects with atrophic gastritis and bacterial overgrowth absorbed significantly less protein-bound vitamin B12 compared to controls, and this was reversed with antibiotic therapy. [17]. Hypochlorhydria is also associated poor iron absorption. The absorption of iron from a typical Western diet by achlorhydric patients is less than physiologic iron losses, creating a negative iron balance. [18]
Why is her Zonulin Elevated?
Some final points to make about this patient: her Zonulin is clearly elevated, at 162ng/g (<107ng/g). This observation is more common in H pylori cases – even when found in low levels such as here, and suggests the H pylori is active in its ulcer and gastric fibrosis inducing activity.
What else can induce elevated Zonulin? Low probiotics levels (Lactobacillus, Akkermansia, Bifidobacteria, Faecalibacterium) which is not so in her case. Higher levels in the autoimmune disease category of the GI Map are more likely to induce zonulin owing to their stronger inflammatory characteristics. This patient has none of those types showing in that section of her GI Map.
However, the patient reported she was often constipated (BM every 2-3 days), with a weekly bout of loose stool. Her constipation could have been partly owing to the excessive methanogenic bacteria found. Methane readings over 12ppm in the breath test are an indication of this, and hers were at 22ppm.
What About The Methane?
In this patient’s GI Map, we can see the Methanogens are at 1.6e9 (1.6 billion), and the labs cut of point is 5e9 or 5 billion. At our clinic we have seen many results where methane in a breath test is found to be >12ppm at anything more than 100 million or 1.0e8 on the GI Map. Read the GI Map carefully in regard to methanogens, over 108 is suspicious and anything in 109 is very likely to have excessive methanogens. Methane/methanogens generally appear in excessive fermentation cases.
Diverticulitis and constipation [19] may occur more frequently in such cases. A meta-study on methane positive found methane positive SIBO in IBS was significantly more prevalent in IBS-C as compared to IBS-D (OR = 3.1, 95% CI 1.7–5.6, P = .0001) – when using the SIBO breath test as the diagnostic tool. [20].
Digestive Dysfunction Dysbiosis
The wider point in this case is how digestive dysfunction is very commonly induced by underactive stomach function initiated by H pylori. This can cause dysbiosis and excessive fermentation from undigested food. Hypochlorhydria, SIBO, pancreatic insufficiency may be initiated even by low H pylori prevalence, especially if Streptococcus (usually found along with Enterococcus, Staphylococcus, Enterobacter) are found in excess.
Summary
Since H pylori is found in half the adult population, and since it can cause hypochlorhydria even in the absence of atrophic gastritis, the presence of H pylori in the hundreds per gram of stool qPCR test results needs to be carefully assessed as a likely cause of digestive dysfunction dysbiosis.
Treatment Concepts
Correction needs to include treatment of the H pylori as the initial step in correction of the related SIBO. Failure to remove H pylori can result in relapse of SIBO and digestive dysfunction dysbiosis. Since the H pylori is overgrowth is mild, herbal plus probiotics treatment is recommended rather than strong triple or quadruple therapy, which may further aggravate the dysbiosis.
After the H pylori and the hydrogen SIBO is managed, then turning to reduce the methanogens is realistic, with replacement enzymes and gut repair formulas likely needed. Some practitioners also use a motility agent for constipated methane patients, but patient compliance in avoiding snacks between meals may be the key to success in permitting natural motility to occur.
Broccoli sprout extracts prevent recurrence of H pylori and may repair GI tissue damage as well. [21]. This and a probiotic are recommended to prevent relapse. Bear in mind 27% of our patients have atrophic gastritis, and this may need ongoing enzyme therapy to compensate, or else relapse of digestive dysfunction dysbiosis may occur – very relevant to older patients especially.
Age and stress are other common causes of digestive dysfunction, so the initial assessment needs a holistic approach to patient care.
Comment On The GI Map
I find the GI Map is the best test for identifying Digestive Dysfunction Dysbiosis. Firstly so many clients in HK have H pylori, which even at low levels can induce this type of dysbiosis. Other tests do not include this or only if added on at significant cost. In my clinic near 25% of individuals tested have digestive dysfunction dysbiosis initiated by H pylori.
Secondly the arrangement of the dysbiosis data on the GI Map is superior to others in allowing easy visualization of dysbiosis patterns such as Digestive Dysfunction Dysbiosis – as well as the insufficiency type dysbiosis.
Finally adding the zonulin really helps with determining if the H pylori is actively causing gastric or duodenal erosion. The only way to be sure the H pylori is removed is another GI Map, as these low levels are not detected by breath tests and are often missed in endoscopic examination as well. You can check correction of the SIBO and the zonulin as well.
The following is a chart outlining all the most common findings of Digestive Dysfunction Dysbiosis. Our patient example showed many of these.
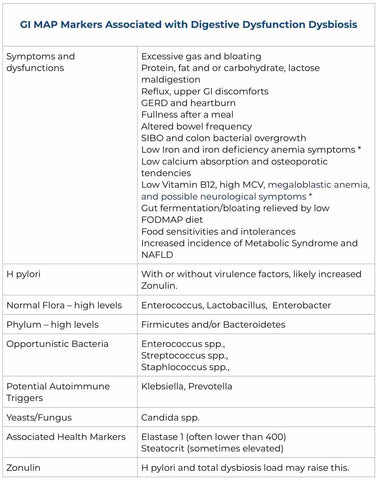
Note:
* Loss of intrinsic factor from atrophic gastritis and SIBO leads to poor absorption of vitamin B12. Signs of atrophic gastritis include vitamin B12 deficiency and megaloblastic anemia (MCV > 90 is suggestive, and >100 indicative), with potential for significant neurological disorders in older patients.
* More commonly malabsorption of iron is evident in hypochlorhydria, leading to iron deficiency anaemia. [22]. Symptomatic patients are mostly females and the related signs of atrophic gastritis are often those associated with iron deficiency: fatigue, restless legs syndrome, brittle nails, hair loss, impaired immune function, and impaired wound healing. [23]
References
1. PMID: 28782119
2. PMID: 12845343
3. PMID: 12845343
4. PMID: 28124156
5. PMID: 20581234
6. PMID: 29316555
7. PMID: 31990420
8. PMID: 21128930
9. PMID: 12845343
10. PMID: 31517648
11. https://doi.org/10.1016/j.hpb.2019.10.1236
12. PMID: 30631759
13. https://doi.org/10.1186/s40168-019-0704-8
14. PMID: 23997926
15. PMID: 26527169
16. PMID: 26527169
17. PMID: 1889697
18. PMID: 25994564
19. PMID: 27129485
20. doi.org/10.1080/19490976.2021.1933313
21. DOI: 10.1158/1940-6207.CAPR-08-0192
22. PMID 12631657
23. PMID 27671008